Chemical Kinetics Class 12 Ncert Solutions Pdf Download
NCERT Solutions for Class 12 Chemistry Chapter 4 Chemical Kinetics- Students appearing in the Class 12 board exam must check these NCERT Class 12 Chemistry Chapter 4 solutions. NCERT solutions for class 12 chemistry chapter 4 Chemical Kinetics covers all the questions from NCERT books for Class 12 Chemistry.
In NCERT Class 12 Chemistry solutions chapter 4, there are questions and solutions of some important topics like average and instantaneous rate of a reaction, factors affecting the rate of reaction, integrated rate equations for zero and first-order reactions, etc. Read further to know all the NCERT solutions for class 12 chemistry chapter 4 Chemical Kinetics.
Check NCERT Class 12 solutions for other subjects.
Rate of reaction- It is defined as the rate of change in concentration of reactant or product. Unit of rate is 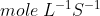 .
.
Reactants, R Products, P
nA+mB pC+qD
NCERT solutions for class 12 chemistry chapter 4 Chemical Kinetics -
Below ate the solutions for exercises 4.1 to 4.9
Question 4.7 What will be the effect of temperature on rate constant ?
Answer :
The rate constant of the reaction is nearly doubled on rising in 10-degree temperature.
Arrhenius equation depicts the relation between temperature and rates constant.
A= Arrhenius factor
Ea = Activation energy
R = gas constant
T = temperature
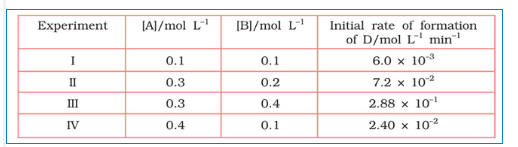
Question 4.11 The following results have been obtained during the kinetic studies of the reaction:
2A + B C + D
![]()
Determine the rate law and the rate constant for the reaction .
Answer :
Let assume the rate of reaction wrt A is and wrt B is
. So, the rate of reaction is expressed as-
Rate =
According to given data,
these are the equation 1, 2, 3 and 4 respectively
Now, divide the equation(iv) by (i) we get,
from here we calculate that
Again, divide equation (iii) by (ii)
from here we can calculate the value of y is 2
Thus, the rate law is now,
So,
Hence the rate constant of the reaction is
Topics and Sub-topics of NCERT Class 12 Chemistry Chapter 4 Chemical Kinetics
- 4.1 Rate of a Chemical Reaction
- 4.2 Factors Influencing Rate of a Reaction
- 4.3 Integrated Rate Equations
- 4.4 Temperature Dependence of the Rate of a Reaction
- 4.5 Collision Theory of Chemical Reactions
NCERT Chapter-wise Solutions for Class 12 Chemistry
More about Class 12 Chemistry Chapter 4 NCERT solutions
-
A total of 5 marks of questions will be asked in the class 12 CBSE board exam of chemistry from this chapter.
-
In this chapter, there are 30 questions in the exercise and 9 questions that are related to topics studied.
-
To clear doubts of students, the NCERT solutions for class 12 chemistry chapter 4 Chemical Kinetics are prepared in a comprehensive manner by subject experts.
-
This chapter is vital for both CBSE Board exam as well as for competitive exams like JEE Mains, VITEEE, BITSAT, etc. so, students must pay special attention to the concepts of this chapter.
-
The NCERT solutions provided here are completely free and you can also download them for offline use also if you want to prepare or any other subject or any other class.
-
By referring to the NCERT Solutions for Class 12 chemistry chapter 4 PDF download, students can understand all the important concepts and practice questions well enough before their examination.
NCERT Solutions for Class 12 Subject wise
-
NCERT Solutions for Class 12 Biology
-
NCERT solutions for class 12 Maths
-
NCERT solutions for class 12 Chemistry
-
NCERT Solutions for Class 12 Physics
Benefits of NCERT solutions for class 12 chemistry chapter 4 Chemical Kinetics
-
First, the easy steps given in the NCERT Class 12 Chemistry solutions chapter 4 will help you to understand the chapter easily.
-
The revision will be so easy that you always remember the concepts and get very good marks in your class.
-
Homework will be easy now, all you need to do is check the detailed CBSE NCERT solutions for class 12 chemistry chapter 4 Chemical Kinetics and you are good to go.
-
If you have a doubt or question that is not available here or in any of the chapters, contact us. You will get the answers with solutions that will help you score well in your exams.
Also check NCERT Exemplar Class 12 Solutions
-
NCERT Exemplar Class 12th Maths Solutions
-
NCERT Exemplar Class 12th Physics Solutions
-
NCERT Exemplar Class 12th Chemistry Solutions
-
NCERT Exemplar Class 12th Biology Solutions
-
NCERT Exemplar Class 12 Chemistry solutions Chapter 4
Solutions
Frequently Asked Question (FAQs) - NCERT solutions for class 12 chemistry chapter 4 Chemical Kinetics
Question: What are the important topics of this chapter?
Answer:
Important topics of this chemical kinetics are the rate of reaction, concept of collision theory, effect of temperature in activation energy, Arrhenius equation, concept of collision theory.
Question: What is the weightage of NCERT class 12 Chemistry chapter 4 in CBSE board exam?
Answer:
The weightage of NCERT class 12 Chemistry chapter 4 in CBSE board exam is 5 marks.
Question: What is the weightage of NCERT class 12 Chemistry chapter 4 in NEET?
Answer:
The weightage of NCERT class 12 Chemistry chapter 4 in NEET is 3%.
Question: What is the weightage of NCERT class 12 Chemistry chapter 4 in JEE Mains?
Answer:
Weightage of NCERT class 12 Chemistry chapter 4 in JEE Mains is 4 marks.
Latest Articles
Explore Popular Degree, Branches and Courses
NCERT solutions for class 12 chemistry chapter 4 Chemical Kinetics
Chemical Kinetics Class 12 Ncert Solutions Pdf Download
Source: https://school.careers360.com/ncert/ncert-solutions-class-12-chemistry-chapter-4-chemical-kinetics
0 Response to "Chemical Kinetics Class 12 Ncert Solutions Pdf Download"
Post a Comment