what if 15.7 ml of titrant were required to reach the endpoint?
Lab 9 - Titrations
Purpose
To determine the concentration of acetic acid in vinegar.
Goals
-
1
To perform an acid-base of operations titration. -
ii
To gain experience titrating carefully to a visible endpoint. -
iii
To proceeds experience monitoring a titration with a pH electrode and determining the equivalence point. -
4
To summate the amount of analyte present from the issue of a titration.
Introduction
Many laboratories clarify consumer products to make up one's mind accuracy in the labeling of the product. The very common and simple technique of titration is demonstrated in this experiment. A titration is an analytical procedure in which a reaction is run nether carefully controlled weather. The stoichiometric volume of i reactant of known concentration, the titrant, that is required to react with another reactant of unknown concentration, the analyte, is measured. The concentration of the analyte is determined from the concentration and book of titrant and the stoichiometry of the reaction between them. The experimental setup is shown in Figure i. A buret, which contains the titrant, is calibrated so the volume of solution that it delivers can be determined with high accurateness and precision. Titrant is added to the analyte until the stoichiometric volume of titrant has been added. This is called the equivalence point, at which the volume of titrant delivered by the buret is read. Ordinarily, the book readings are estimated to the nearest 0.01 mL. The delivery of the titrant is adjusted with the stopcock on the buret. With practice, one tin dispense fractions of a drib of titrant and command the process well enough that replicated titrations agree within 0.10 mL. For this first lab, you volition demand your titrations to concur to within 0.50 mL. Effigy 1 : Titration Setup ( 1 ) HC2H3Oii(aq) + OH–(aq) → CiiH3O2 –(aq) + HtwoO(l) ( 2 ) moles HC2H3O2 reacting = moles OH– added ( 3 ) moles of acid reacting = moles of base reacting ( 4 ) 10.two mL solution × (Macid = molesacid / Fiveanalyte) ( 5 ) Mass % = 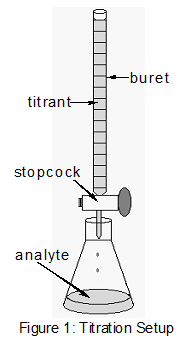
= 1.02 mmol NaOH 0.100 mmol NaOH 1 mL solution
× 100 mass of acetic acrid in sample mass of vinegar solution titrated
Equipment
-
i
MicroLab Interface -
i
MicroLab pH Measurement Pedagogy Sheet -
one
pH electrode in pH 7.00 buffer -
ane
10.0 mL graduated cylinder -
1
30 mL chalice -
one
100 mL chalice -
2
250 mL beakers -
i
25 mL buret -
1
ring stand -
ane
clench -
i
buret clench -
i
magnetic stir plate -
ane
magnetic stir bar -
1
deionized water squirt bottle -
1
box of Kimwipes
Reagents
- ~l mL 0.5 M sodium hydroxide (NaOH)
- commercial vinegar (HCiiH3O2)
- pH 4.00 buffer
- pH 7.00 buffer
- pH 10.00 buffer
- phenolphthalein solution
- deionized water
Safety
NaOH is corrosive. It can attack the skin and crusade permanent impairment to the optics. If NaOH solution splashes into your eyes, employ the eyewash immediately. Agree your optics open and flush with water. If contact with peel or vesture occurs, affluent the affected surface area with water. Take your lab partner notify your instructor about the spill.
Waste Disposal
All solutions tin can be flushed downwards the sink with plenty of water.
Prior to Form
Please read the following sections of the Introductory Textile:
Delight review the following videos:
Please complete your WebAssign prelab consignment. Check your WebAssign Account for due dates. Students who do non complete the WebAssign prelab assignment are required to bring and hand in the prelab worksheet.
Lab Procedure
Please print the worksheet for this lab. You volition need this sheet to record your information.

In this experiment, you will be using pH electrodes connected to the MicroLab Interface. pH electrodes have a thin glass seedling at the tip. They interruption easily and are plush to supersede. Exist careful non to shove the electrode into the bottom of a chalice or drop the electrode. There is a protective guard around the tip, which should remain in identify at all times. The guard volition not protect against devil-may-care treatment. Delight utilise farthermost care when using this equipment. Best results in using the electrodes are obtained if: • • •
Part A: Calibrating the MicroLab pH Electrode
-
i
Open up the MicroLab program. -
ii
Make sure the pH electrode is plugged into the interface. -
3
Calibrate the pH electrode using the MicroLab instructions provided in the lab. -
4
Configure the MicroLab program using the education sheet provided. -
5
Later on the calibration and configuration are complete, measure out the pH of each of the 3 buffer solutions of pH = 4.00 (cerise), pH = 7.00 (yellow), and pH = x.00 (blue). Record the value in the digital brandish into WebAssign as a tape of how accurately the probe is calibrated. Make sure the electrode is immersed in the solution and let for a few seconds equilibration.
Office B: Titration of Vinegar Monitored by pH Probe and Indicator
-
ane
Obtain a clean, dry 10.0 mL graduated cylinder. -
2
Using a make clean, dry thirty mL beaker, obtain about 25 mL of vinegar. -
3
Condition the graduated cylinder with vinegar solution before using it. This is done past adding a petty vinegar solution to the graduated cylinder, swirl and then that all of the sides are coated with vinegar and then discarding the remaining vinegar. Echo this procedure 1–two more than times to ensure that the graduated cylinder is conditioned. -
4
Measure the mass of an empty 250 mL beaker and record this value in Data Table A1. Using the 10.0 mL graduated cylinder, transfer vii.0 mL of vinegar into the beaker. Weigh the chalice and vinegar together and record the mass in Data Tabular array A1. Tape the volume of vinegar in Data Table A1 as well. -
5
Add ~40 mL of deionized water (exercise not use the graduated cylinder for this now that it is conditioned for vinegar!) and iii drops of phenolphthalein solution to the beaker containing the vinegar. -
vi
Obtain virtually 50 mL of ~0.five K NaOH solution in a clean, dry out 100 mL chalice. Record the verbal concentration from the canteen of NaOH in Data Table A1. -
7
Status the 25.0 mL buret with NaOH solution as directed past your teacher, and according to the description in "Volumetric Glassware" in Lab Equipment. -
8
Fill the buret with NaOH and advisedly clamp information technology to the band stand. Make sure to fill up the tip with NaOH solution by draining some solution from the tip into a waste chalice. For this experiment, the titration volumes volition exist easier to enter into the MicroLab software if the starting volume of NaOH is EXACTLY 0.00 mL. -
ix
Carefully slide the stir bar into the 250 mL chalice containing the vinegar solution while tilted to avoid splashing or damage to the beaker. Position the stir plate under the 250 mL beaker and begin stirring slowly. -
10
Carefully position the pH electrode in the 250 mL breaker until nigh ane/ii inch of the tip is in the solution. Clamp to the ring stand with the clench provided. Be sure that the stir bar will not strike the pH electrode. If necessary, add more than h2o. See Figure 2 for the complete setup.
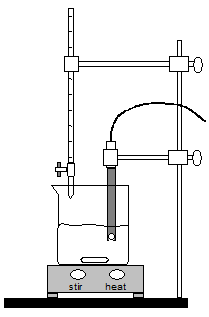
Figure two : Experimental Setup
-
eleven
Position the buret and then that the tip of the buret is just inside the beaker. Refer to Effigy 2. -
12
Have an initial pH reading by inbound the initial buret reading in the MicroLab software window and hitting return. You should as well record all of your data in Data Tabular array B simply in instance something goes wrong with the computer. Remember to read the buret to the nearest 0.01 mL . Reading a buret to this accuracy is catchy; the last meaning effigy is expected to exist an guess.
Figure 3 : Data Tabular array B: Volume of Titrant Added to Vinegar vs pH
-
13
Open the stopcock of the buret and add ~2.0 mL of titrant (NaOH) to the contents of the beaker (HC2HthreeO2 + h2o + indicator). Stir about 10 seconds. So read the exact volume on the buret, enter this value into the MicroLab software and accept a pH reading. Remember to tape your measurements in Data Table B. -
fourteen
Proceed to add together titrant in ~2.0 mL increments and record the buret volume and pH in Data Table B. Finish at ~viii.0 mL of titrant volume. -
fifteen
After ~eight.0 mL of titrant has been added, the increment of titrant improver should be decreased as the endpoint is closer. Add together titrant in ~1.0 mL amounts until a total of ~11 mL of titrant has been added. Then reduce the amount of titrant addition to ~0.5 mL increments or less. At this point, the pH should modify more than than 0.3 pH units per add-on, signaling the titration endpoint. You will also run across a faint pinkish color appear and apace fade. When the colour begins to disappear more slowly, tedious the addition of titrant to a dropwise rate. Rinse the walls of the chalice and the tip of the buret with deionized water from a wash bottle as you lot approach the endpoint. This ensures that all of the NaOH delivered from the buret ends up in the reaction mixture. The endpoint has been reached when the faint pink color lasts for at least xxx seconds. Tape the equivalence betoken reading on the buret to the nearest 0.01 mL in Data Table A1. -
16
To finish generating the titration curve, render to 1 mL increments of titrant as the changes in pH decrease below 0.iii pH units across the equivalence point. Do not finish the titration until yous have added approximately 5 mL of titrant across the equivalence point. -
17
When you lot are finished with your titration, stop the MicroLab information drove plan. Carefully remove the pH electrode from the solution, rinse it off and identify it in the pH 7 buffer. -
18
View the graph generated by the MicroLab titration program to become familiar with the appearance of a typical titration bend. Echo steps 1–thirteen with a second sample of vinegar. Information technology is not necessary to condition glassware a 2nd fourth dimension. -
xix
When finished, drain the remaining NaOH from your buret into your 100 mL beaker. Discard all solutions in the sink with plenty of water. -
20
Rinse all of your glassware with water, dry out it and return it to the prepare-up area where y'all constitute it. Close the MicroLab program.
Data Table A1: Experimental Information
Question 1: The titration bend of a weak acid like acetic acrid with base has a distinctive advent when the volume of titrant is plotted on the x-axis and the pH is plotted on the y-axis. Select the picture that most closely resembles this graph.
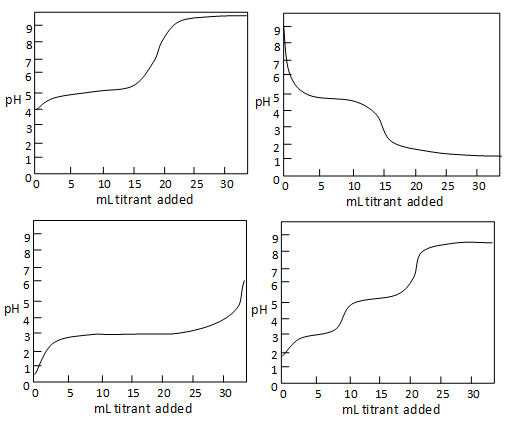
Figure 4
Question 2: What is the color of the solution at below pH 8? What is the color of the solution in a higher place pH 8? Observe pH 8.00 on your titration graph. How shut is the amount of titrant at pH 8.00 to the Equivalence Indicate Buret Reading? Inside 0.l mL? Within i.00 mL?
Question 3: Calculate the number of millimoles of NaOH required to accomplish the endpoint for each of the three titrations. Show one adding completely. What is the average? Record the values in Data Table A2.
Question 4: How many millimoles of acetic acid are in each vinegar sample? Bear witness ane calculation completely. What is the average? Tape the values in Information Table A2.
Question 5: What is the mass of acerb acid in each vinegar sample? Prove one calculation completely. What is the average? Record the values in Data Table A2.
Question 6: What is the molarity of acetic acid in each vinegar sample? Bear witness one calculation completely. What is the average? Record the values in Data Table A2.
Question 7: What is the mass % of acetic acid in each vinegar sample? Show ane adding completely. What is the boilerplate? Record the values in Information Table A2.
Data Table A2: Calculated Results
Question eight: Do yous adopt monitoring a titration with a pH probe or an indicator? Explain your selection.
-
21
Earlier leaving, go to a figurer in the laboratory and enter your results in the In-Lab consignment. If all results are scored as correct, log out. If non all results are correct, try to observe the error or consult with your lab instructor. When all results are right, note them and log out of WebAssign. The In-Lab assignment must be completed by the end of the lab period. If additional time is required, please consult with your lab teacher.
Source: https://www.webassign.net/question_assets/ncsugenchem102labv1/lab_9/manual.html
0 Response to "what if 15.7 ml of titrant were required to reach the endpoint?"
Post a Comment